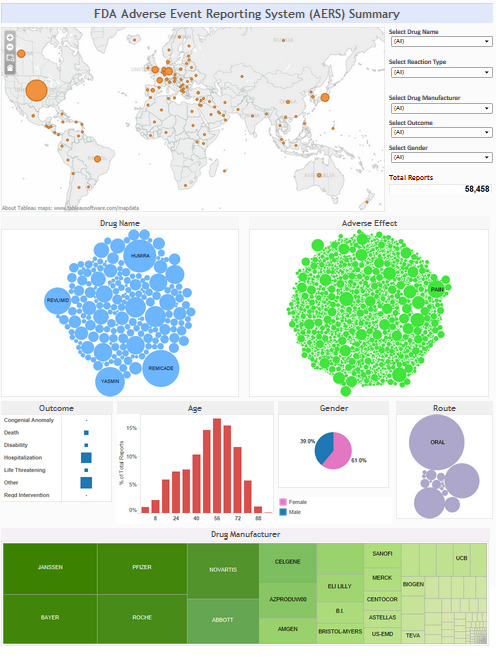
Food and Drug Administration – AERS
The FDA oversees the safety and efficacy of all over-the-counter and prescription drugs marketed and sold in the United States. With as many as 3 billion prescriptions written annually coupled with the inherent risks of drug use, close to one million adverse events were reported to the FDA in 2011.
Improving our understanding of adverse reactions to drugs can considerably reduce not only injuries, deaths and healthcare cost, but can significantly improve safe patient care and public health. Mathematical techniques including data mining can be used for signal detection, finding statistical associations and patterns between drug products and adverse events.
Objectives
- Create an dynamic, interactive dashboard that provides insight of potential safety signals
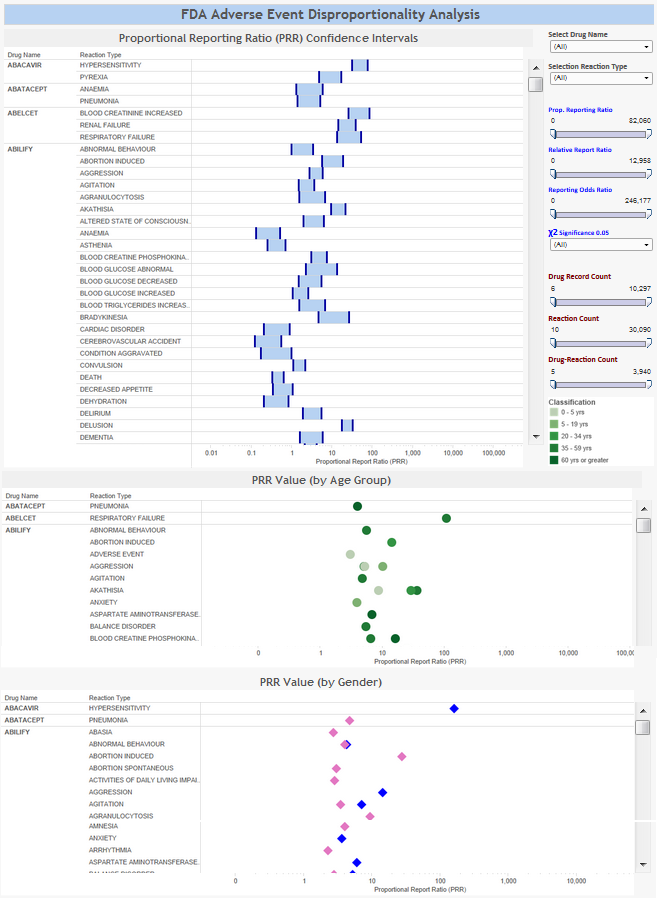
- Identify strong signals between drugs and adverse events that may warrant further investigation using:
- Disproportionality Analysis: Proportional Reporting Ratio (PRR), Relative Reporting Ratio (RRR), Reporting Odds Ratio (ROR)
- Independence Testing: Standard errors, confidence intervals, and chi-squared (χ2) values
- Stratification Analysis: Confounding factors (age and gender)
Challenges
Some of the challenges of conducting data analysis on FDA’s AERS data files included duplicate reporting, reporting bias, inconsistent reporting, and missing data.
Visualizations
Click on the images below to view the dashboards.